Day 1 :
Keynote Forum
Gabriel Kristian Pedersen
Statens Serum Institut, Denmark
Keynote: Targeted delivery in rational vaccine design
Time : 8:30-9:10

Biography:
Gabriel Pedersen is an immunologist and head of section for the Adjuvant Research group at the Center for Vaccine research, Statens Serum Institut, Copenhagen. Gabriel did his PhD at University of Bergen, Norway, focusing on pandemic influenza vaccines, before moving to Karolinska Institutet, Sweden, to do a postdoc in B cell biology, with particular focus on innate-like B cells. Since 2016, he has focused on adjuvant research on SSIs novel pipeline of adjuvants, particularly focusing on liposome and emulsion-based adjuvant and delivery systems.
Abstract:
Novel vaccine strategies include the so-called subunit vaccines, which encompass only the part of the pathogen to which immune recognition results in protection. The high purity of these vaccines makes adverse events less likely, but it also makes the vaccines less immunogenic and therefore potentially less effective. Vaccine adjuvants that increase and modulate the immunogenicity of the vaccine are therefore added to solve this problem. Besides aluminum salts, which have been used in vaccines for 90 years, a number of novel vaccine adjuvants include delivery systems like liposomes and emulsions have been included in licensed vaccines over the last 30 years. However trial-and-error has been the explorative approach of choice, for the design of novel vaccine adjuvants due to major gaps in the knowledge about immunological activation processes. Increasing insight into immunological mechanisms and how to manipulate them has replaced empirical with rational design of adjuvants, leading to vaccine adjuvants with increased and customized immunogenicity profiles without compromising vaccine safety. I will present an overview of where vaccine adjuvant research is today. I will furthermore show an example where the newest knowledge in innate immunology enables the rational design of a novel CTL inducing vaccine adjuvant.
Keynote Forum
Carmen Alvarez-Dominguez
Instituto de Investigación Marqués de Valdecilla, Spain
Keynote: Listeria based nanovaccines as therapeutic vaccines
Time : 9:15-10:00

Biography:
Carmen Alvarez-Dominguez has completed her PhD in Immunology, 1993 has her expertise in listeriosis and Listeria based vaccines and nano-vaccines for biomedical purposes. Her group has prepared different vaccines for listeriosis, either systemic listeriosis or neonatal listeriosis, using different vectors such as dendritic cells or nanoparticles. Moreover, they have also prepared Listeria-based nano-vaccines as therapeutic tools for solid tumours. She has built this vaccine expertise after more than 27 years of experience in research, evaluation and teaching in hospital, basic research and academic institutions in Spain and USA. She is also moving recently to consultancy companies to put new vaccines into the market.
Abstract:
Dendritic cell-based (DC-based) vaccines are promising immunotherapies for cancer. However, several factors, such as the lack of efficient targeted delivery and the sources and types of DCs, have limited the efficacy of DCs and their clinical potential. We propose an alternative nanotechnology-based vaccine platform with antibacterial prophylactic abilities that uses gold glyconanoparticles coupled to listeriolysin O 91–99 peptide (GNP-LLO91–99), which acts as a novel adjuvant for cancer therapy as well as therapeutic vaccine for cutaneous melanoma acting as a novel immunotherapy. GNP-LLO91–99 exhibited dual anti-tumour activities, namely, the inhibition of tumour migration and growth and adjuvant activity for recruiting and activating DCs, including those from melanoma patients. GNP-LLO91–99 nanoparticles caused tumour apoptosis and induced antigen and melanomaspecific cytotoxic Th1 responses (P≤0.5). They also cause tumour complete remission and survival improvement. GNP-LLO91-99 nanovaccines presented superior tumour rejection and survival benefits, when combined with anti-PD-1 or anti-CTLA-4 checkpoint inhibitors, predicting an improvement of these immunotherapies action. Studies with monocyte-derived DCs of patients with stage IIIB melanoma confirmed the ability of GNP-LLO91-99 nanovaccines to complement the action of check point inhibitors, not only reducing cell-death markers expression on DCs, but also potentiating DC antigen-presentation and production of Th1-Th12 cytokines. We propose that GNP-LLO91-99 nanovaccines function as immune stimulators and immune effectors and serve as safe cancer therapies, alone or in combination with other immunotherapies.
Keynote Forum
Mario A Bianchet
Johns Hopkins School of Medicine, USA
Keynote: Overcoming antibiotic resistance: Inhibition of ld-transpeptidation in multi-drug resistant pathogens
Time : 10:20-11:00

Biography:
Mario A Bianchet has completed his PhD from National University of La Plata, Argentina and performed his Postdoctoral training at Johns Hopkins School of Medicine where he joined as Assistant Professor in the Department of Neurology in 2011. He is a Structural Enzymologist and has participated in several seminal structural and mechanistic studies of macromolecular systems of biomedical interest including cell-wall biosynthesis: LD-transpeptidases and their complexes with carbapenems. In addition, he has valuable contributions to diverse fields, including bioenergetics: F1-ATPase, xenobiotic-response: NADPH:Quinone oxidoreductases, DNA-repair: Uracyl-glycosylase/inhibitors, carbohydrate-recognition: animal lectins, resulting in 70 publications in reputed journals.
Abstract:
Multidrug-resistant microorganisms produce infections that are hard to treat or may even be untreatable with conventional antimicrobials. Mycobacteria tuberculosis (Mtb), Clostridae difficile (Cd), and ESKAPE pathogens are capable of developing resistance to not only clinical antibiotics, but to the last resort (such as carbapenems) as well. The development of novel treatment options to replace antibiotics that lost their effectiveness and new antibiotic targets to circumvent target-specific resistance, which has emerged against every antibiotic class, are high priorities.The development of the necessary new antibiotics through trial-and-error is a costly and time-consuming proposition. Structure-based drug design accelerates discovery by linking structural information and computational techniques. The targeting late stages of bacterial cell-wall biosynthesis remains a sound strategy. The peptidoglycan of Mtb, M. abscessus, and Cd during stationary growth is synthetized by LD-transpeptidases (LDTs), different enzyme than DD-transpeptidases (Penicillin-binding proteins) the primary target of β-lactams. This difference contributes to these pathogens resistance to β-lactams. We are investigating the molecular structures of Mtb LDTs and its complexes with carbapenems. Structural evidence from these studies suggests that the catalytic site flexibility dynamically accommodates ligands larger that than the geometric volume of the site observed in the crystallographic structure. Thus, inhibitors binding to LDTs can involve transient active-site conformations unobserved in the time-averaged crystallographic structure. We are developing methods to seek LDTs inhibitors targeting accessible dynamic states of these enzymes in drug-resistant pathogens to obtain antibiotic leads. In this talk, I’ll present preliminary results of our work targeting essential Mtb transpeptidase, LdtMt2
Keynote Forum
Marek Chmielewski
The Polish Academy of Sciences, Poland
Keynote: Kinugasa reaction as an attractive method of the synthesis of β-lactam drugs
Time : 11:20-12:00

Biography:
Marek Chmielewski completed his PhD from the Institute of Organic Chemistry, Polish Academy of Sciences and Post-doctoral studies from Purdue University and SIU Carbondale (USA). He has been the Director of the Institute of Organic Chemistry (2004–2010), and Vice-President of the Polish Academy of Sciences (2011–2014). He has published more than 250 papers in reputed journals
Abstract:
The copper(I) mediated reaction of nitrones and terminal acetylenes, which is known as Kinugasa reaction, represents an attractive tool of direct formation of the β-lactam ring. The attractiveness of this reaction includes the use of readily available starting materials, high functional group tolerance, high atom economy, and relatively high stereochemical control of the reaction pathway. We present our studies on the application of Kinugasa reaction in synthesis of: carbapenems thienamycin, Fig 1a) and 4AA azetidinone, Fig 1b), monobactams carumonam, Fig. 1c), aztreonam, Fig 1d), and ezetimibe, a powerful cholesterol absorption inhibitor, Fig. 1e).
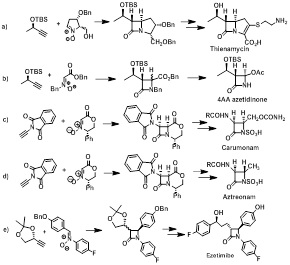
Figure 1: Substrates of Kinugasa reactions, adducts and target products.
