
Marek Chmielewski
The Polish Academy of Sciences, Poland
Title: Kinugasa reaction as an attractive method of the synthesis of β-lactam drugs
Biography
Biography: Marek Chmielewski
Abstract
The copper(I) mediated reaction of nitrones and terminal acetylenes, which is known as Kinugasa reaction, represents an attractive tool of direct formation of the β-lactam ring. The attractiveness of this reaction includes the use of readily available starting materials, high functional group tolerance, high atom economy, and relatively high stereochemical control of the reaction pathway. We present our studies on the application of Kinugasa reaction in synthesis of: carbapenems thienamycin, Fig 1a) and 4AA azetidinone, Fig 1b), monobactams carumonam, Fig. 1c), aztreonam, Fig 1d), and ezetimibe, a powerful cholesterol absorption inhibitor, Fig. 1e).
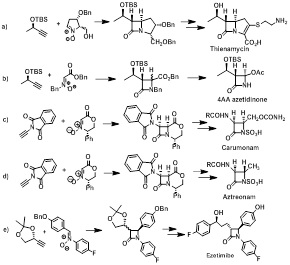
Figure 1: Substrates of Kinugasa reactions, adducts and target products.
