Myung-Soo Kang
iNtRON Biotechnology, South Korea
Title: From discovery to preclinical studyof N-Rephasin® SAL200: A novel antibacterial drug for multidrug-resistant Staphylococci-associated Infections
Biography
Biography: Myung-Soo Kang
Abstract
Statement of the Problem: Emergence and dispersal of multidrug-resistant Staphylococcus aureus (SA) (MRSA) are rapid and wide, necessitating an invention of novel class of MRSA-controlling effective antimicrobial drug that is not likely to induce a resistance. This presentation describes our 15 year-long experience in development of a bacteriophage-derived endolysin as promising new antibiotics.
Materials & Method: A bacteriophage SAP-1 specifically infecting SA was isolated from sewage water. Whole genome sequencing revealed typical genomic structure of lytic bacteriophage and identified putative peptidoglycan (PG) hydrolase gene encoding SAL-1 endolysin. The wild type SAL-1 with no extraneous amino acids was highly purified in compliance to a good manufacturing practice (GMP) standard and further formulated to N-Rephasin® SAL200.
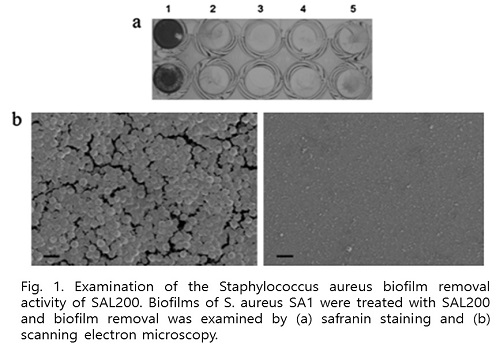
Findings: SAL200 showed to have unique modes of action: It potently and specifically killed planktonic and encapsulated SA as well as biofilm-producing SA cells, but not for other species. SAL200 exhibited strong bactericidal activity against more than 425 clinical isolates including 1 vancomycin-intermediate SA and 336 methicillin-resistant SA isolates. It directly bound to and lysed SA as quickly as within ten minutes and apparently did not induce an emergence of any resistant SA strains from 30-times repeated exposure at sub-lethal concentrations. Intravenous injection of SAL200 significantly prolonged survival of mice and reduced viable bacterial counts in MRSA infection mouse model. Further safety evaluation studies for rodents, dogs and monkeys showed neither death nor severe adverse events. Any abnormal findings-if any- were tolerable and transient.
Conclusion: The SAL200 specifically lysed SA strains including antibiotic-resistant SA strains in vitro and in vivo with excellent in vivo safety.